The Cost to Cure Genetic Disorders
Advances in the gene and cell therapy markets have opened doors to alleviate much suffering caused by genetic disorders. The potential for treatment – or even cure – of so many disease states with little or no current therapy options elicits hope for patients living with these conditions.
However, barriers to accessing gene and cell therapies are largely focused around the following:
• Pricing
• Reimbursement
• Manufacturing
Pricing in the gene and cell therapy line is often ultra-high. With such a large expenditure in research for each disease state, combined with a limited number of patients per disease, a large price tag is often required for manufacturers to receive return on investment.1 For payers, large price tags raise questions around return on investment and reinforce a deeper case for value-based contracting.
The pipeline of proposed therapies suggests $2 million+ will be commonplace as these therapies emerge to market. The huge, one-time, upfront nature of these payments presents economic challenges for many stakeholders, but ultimately for the patient.
Payment Options and Strategies
The long-term savings associated with gene and cell therapies over the course of the life of a patient cured by this treatment is potentially significant.1 However, in the United States, the frequency at which patients change insurers in a private insurance-based market is common. As a result, the long-term savings resulting from a large, upfront payment may not be realized by that particular payer and when the patient changes insurers.2 Another payer concern is a reservation about such high costs for purported cures whose long-term effects of treatment are unproven and efficacy largely unknown at the current time.1
Additionally, approvals for some of these therapies that profess a cure or long-term benefit can be based on single-arm trials with a small number of patients.3 This raises doubt for payers regarding the true length of therapeutic benefit and whether a one-time treatment would actually be sufficient or would require one or more additional treatments over the course of any given patient’s life.3
Given these factors, alternative financing and payment models will most likely be sought out by payers in lieu of large, upfront payments. Many options and strategies have been suggested to thwart traditional payment models, which are likely unsustainable.
Proposed Payment Models
The table below describes numerous financing and payment options currently being discussed for gene and cell therapies. Every option has its own set of pros and cons with the goal of each to grant patients access to these milestone therapies.
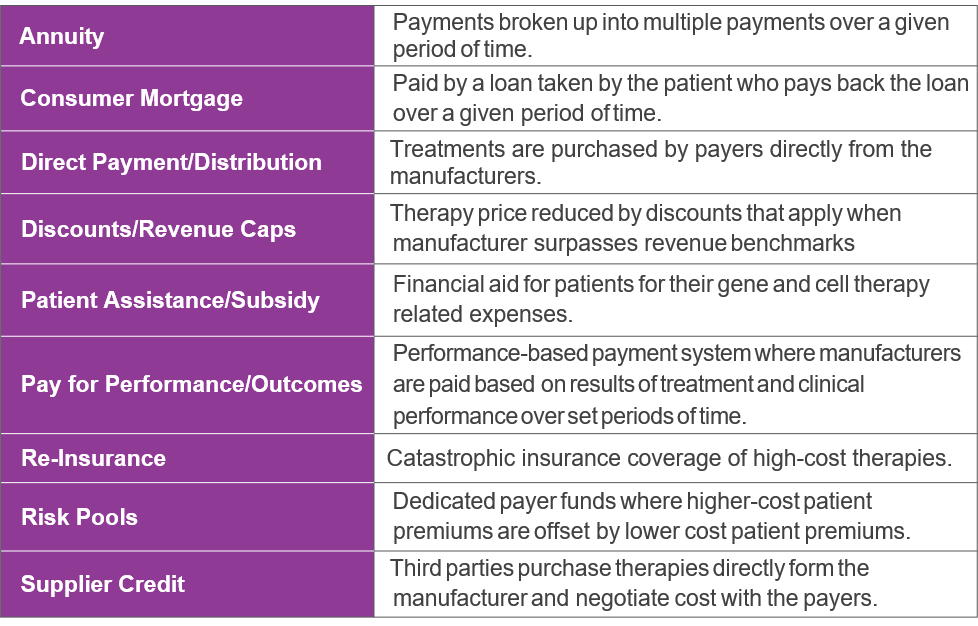
Source: Datamonitor Healthcare payer research; In Vivo, 2017, 2018; Institute for Clinical and Economic Review, 2017; Managed Healthcare Executive, 2018; Penn Medicine News, 2014; Reuters, 2015
The Challenge of Defining Value
An additional challenge for reimbursement is defining value. From a payer perspective, it is easy to only look at cost savings over time. However, people will define value differently, and payers, as a result, may have to include novel metrics that may not normally be applied. As these therapies emerge with greater frequency to cover a broader spectrum of diseases, societal pressure may play a role in valuating therapies.
In an ideal world, where pricing and reimbursement weren’t factors, there are still logistical impediments to the mass commercialization of these treatments. The manufacturing process for these therapies is quite arduous requiring significant investment and time. Most therapies are generated in a patient-specific manner which eliminates manufacturers’ ability to produce in bulk.4 Additionally, the production of viral vectors used to deliver these therapies can seriously delay treatments as many manufacturing facilities creating them have long waits between 12-24 months.3 Without reliable vector production, patient access to gene and cell therapies is reduced.
Specialty Pharmacies Can Help Control Costs
Although gene and cell therapies are usually one-time treatments requiring healthcare provider administration, specialty pharmacies play a critical role in the distribution and cost containment of these cutting-edge products. Specialty pharmacies historically reduce cost versus hospitals and provider centers which historically mark up the cost of therapies received directly from pharmaceutical manufacturers.
Under current arrangements, the contracted specialty pharmacies purchase product from the manufacturer, handle distribution of product for individual patients to healthcare providers and bill the patient’s insurance plan for reimbursement for the drug product.5 Payments to healthcare providers for administration, hospital stays and other care and services related to the therapy are made directly by the health plan. Due to a breadth of experience in areas that may be key to successful deployment of a gene or cell therapy, specialty pharmacies are well-positioned to control cost over many buy and bill models that exist with hospitals and treatment centers.
Researched by Adrienne Brennan, PharmD, CSP, and Marcie Morris, PharmD, CSP, clinical program managers at AllianceRx Walgreens Pharmacy.
Sources
1. Datamonitor. Gene Therapy Commercialization– Opportunities and Barriers. Accessed April 24, 2020 from service.datamonitorhealthcare.com.
2. Institute for Clinical and Economic Review. GENE THERAPY: Understanding the Science, Assessing the Evidence, and Paying for Value. Accessed March 19, 2020 from icer.org/assessment/gene-therapy-2016.
3. Biomedtracker. Gene Therapy: A Paradigm Shift in Medicine. Accessed March 19, 2020 from biomedtracker.com.
4. Institute for Clinical and Economic Review. GENE THERAPY: Understanding the Science, Assessing the Evidence, and Paying for Value. Accessed March 19, 2020 from icer.org/assessment/gene-therapy-2016.
5. How Express Scripts Plans to Disrupt the Emerging Cell and Gene Therapy Channel. Drug Channels. Published on October 10, 2019. Accessed on April 24, 2020. Available at: drugchannels.net/2019/10/how-express- scripts-plans-to-disrupt.html
Back to Blog > Blog Stories




