Vectors: The Cornerstone of Gene Therapies

When a patient has a genetic disease, it means there is a spontaneous or inherited mutation (change) in a person’s DNA. These mutations result in the production of proteins with limited or no function.1 Depending on which genes and proteins are affected, they can have minor or catastrophic consequences, including rare genetic disease. Historically, for the vast majority of patients with rare genetic diseases, very few or possibly no treatments were even available. Even if a treatment for a particular disease was available, it would likely only treat the symptoms. These patients had little hope and a very poor prognosis.
How Gene and Cell Therapy Works
As modern medicine has developed, new types of treatment called gene and cell therapies have become a major focus of the biopharmaceutical industry. The goal of these exciting new treatments is not only to treat diseases with no current options, but in many cases to actually cure patients of their disease. While the goals of both gene therapy and cell therapy are the same, the mechanisms by which each work are decidedly different.
In cell therapy, scientists use cells from the patient or a donor and grow or modify them outside of the body.2 These cells are specific to each patient and contain a “normal” copy of the gene in which that particular patient has a mutation. During treatment, a medical professional injects these cells into the patient. Once inside, these cells then begin to make the functional protein a patient’s DNA cannot produce due to the mutation. By contrast, gene therapy entails treating diseases at the genetic level of the patient by either replacing, deactivating or repairing the faulty DNA that is causing the disease.1 Through a vector, a medical professional delivers into a patient this new, functional DNA which is swapped out for the mutated DNA.
Special Delivery by Vector
To get DNA into a patient’s cells, something must deliver the new, working genetic material to the cells in the body. This delivery mechanism is known as a vector. Many different vessels can be used as vectors and they often fall into two types, viral and non-viral.3 The ideal vector and delivery system depends on the target cells and its characteristics, duration of expression and the size of the genetic material to be incorporated in the vector.4 There are advantages and disadvantages to each which can be seen in the chart below.
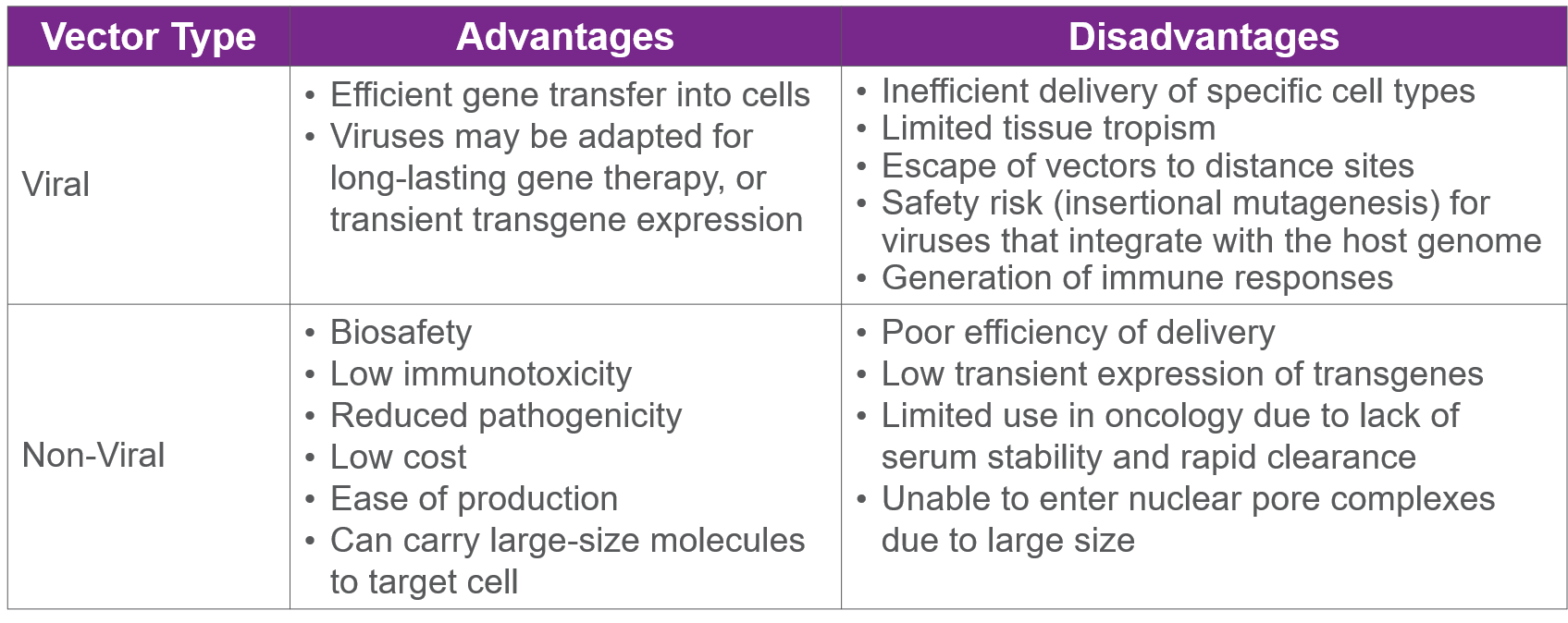
Source: Nayak and Herzog, 2010; Ramamoorth and Narvekar, 2015; Seow and Wood, 2009; Zhang et al., 2012
Non-viral vectors can include cells, bacteria, messenger RNA, liposomes and plasmids. The biggest perks of non-viral vectors are that they are less expensive than viral vectors to produce, and have low immunotoxicity and pathogenicity. One major drawback to non-viral vectors is their inefficient delivery.3
Due to their ability to naturally infect cells, viruses –such as adeno-associated viruses (AAV) and lentiviruses –are the most common vector of choice in both approved treatments and those currently being developed. Viral vectors account for approximately 59% of gene therapy prospects.3 The viruses used as vectors, which are either known to not cause disease or have been genetically modified in a lab to not cause disease, carry the DNA into the host cell and insert the material directly into the patient’s DNA. With functional DNA restored, cells can now properly produce proteins. As a result, the severity of the disease decreases and may be potentially completely cured.
As you can see, despite it often being referred to as a single technology, there are different approaches to gene therapy, including the type of vector chosen for delivery.
References
1. Institute for Clinical and Economic Review. GENE THERAPY: Understanding the Science, Assessing the Evidence, and Paying for Value. Accessed March 19, 2020 icer.org/wp-content/uploads/2020/11/ICER-Gene-Therapy-White-Paper-030317.pdf
2. Novartis. What is cell and gene therapy. Accessed March 19, 2020 from novartis.com/our-focus/cell-and-gene-therapy/what-cell-and-gene-therapy
3. Datamonitor Healthcare. Gene Therapy Pipeline and Portfolio Analysis. Accessed April 3, 2020 from service.datamonitorhealthcare.com (account required for access)
4.Non Viral Vectors in Gene Therapy- An Overview. Accessed August 8, 2021 from ncbi.nlm.nih.gov/pmc/articles/PMC4347098
Back to Blog > Blog Stories



